by Yekta Dowlati, PhD
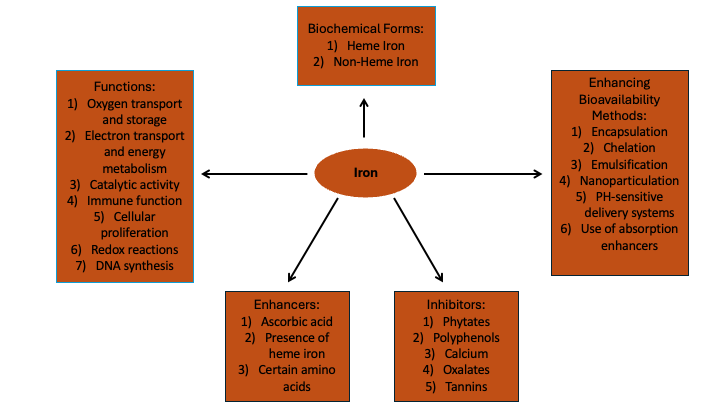
Iron is a vital nutrient that serves as a fundamental component of heme and various coenzymes, playing critical roles in essential biochemical pathways. It is integral to the transport of oxygen via hemoglobin, supports mitochondrial oxidative phosphorylation for energy production, and is necessary for the synthesis of DNA.1
In female individuals, physiological changes during the menstrual, gestational, and lactational phases result in dynamic requirements for iron homeostasis. Sufficient iron consumption is essential for maintaining overall health and preventing conditions such as iron deficiency anemia (IDA), which impacts a considerable number of women globally.
This blog post examines the critical role of iron in women’s health, specifically addressing the daily iron necessities across various life stages and the iron supplementation options available. Additionally, it evaluates the factors that affect iron absorption and outlines methods to improve the efficacy of iron supplementation, highlighting the need for personalized strategies to optimize patient care.
The established daily iron requirement for premenopausal women is 18 mg to offset menstrual blood loss. The National Institutes of Health (NIH) state that menstruation leads to an average iron loss of about 1 mg/day in women with regular cycles. The need increases to 27 mg/day during gestation to accommodate hematopoietic expansion, fetal needs, and peripartum reserves.2 Concurrently, the World Health Organization (WHO) targets a 50% reduction in the prevalence of anemia among women by 2025, highlighting the global urgency of addressing iron deficiency.3 The WHO advises a daily intake of 30 to 60 mg of elemental iron during pregnancy to prevent maternal anemia and its associated risks such as puerperal sepsis, low birth weight, and preterm birth.4 The recommended dietary allowance (RDA) for iron dips to 9 mg/day postpartum to account for lactation demands, notwithstanding the initial amenorrhea phase, yet remains elevated to replenish maternal iron reserves for milk synthesis.5 In postmenopausal women, the iron requirement decreases to 8 mg/day, mirroring that of males due to the cessation of menstrual blood loss.2
In 2016, IDA was estimated to affect over 1.2 billion individuals globally, signifying its status as a primary public health challenge. The prevalence of IDA is notably higher among specific demographics, including 41.7% of children under five, 40.1% of pregnant women, and 32.5% of non-pregnant women.6 Furthermore, the incidence of iron deficiency varies across different racial and ethnic groups, with higher rates observed in Hispanic and black women compared to their white and Asian counterparts.7
Bioavailability: Iron Supplements
The bioavailability and efficacy of iron supplementation depends on the chemical form of the administered iron and its interaction with the gastrointestinal environment. Ferrous salts (sulfate, gluconate, fumarate) are conventionally utilized due to their superior intestinal absorption compared to ferric forms.8 However, the ionic state, solubility, and gastrointestinal pH significantly modulate their bioavailability. Ferrous sulfate, the most prescribed form, offers high elemental iron content but may induce oxidative stress and mucosal irritation in susceptible individuals. Ferrous gluconate, with lower elemental iron content, provides a gentler alternative, however, achieving equivalent efficacy may require administrating larger dosages. Ferrous fumarate represents a middle ground, balancing elemental iron content with patient tolerability, beneficial in pronounced deficiency states. Intravenous ferric carboxymaltose is a treatment option for recalcitrant anemia or malabsorption syndromes, providing high-dose iron replenishment with minimal gastrointestinal side effects.9 Recently, the focus has shifted towards complex forms of iron such as iron bisglycinate chelates, which is designed to reduce gastrointestinal side effects and enhance absorption through bypassing traditional ionic pathways.10 These advanced formulations leverage the mucosal cell’s transporter mechanisms, offering a novel approach to improving systemic iron bioavailability while minimizing adverse gastrointestinal interactions. This nuanced understanding of iron pharmacokinetics underscores the importance of individualized supplementation strategies to optimize therapeutic outcomes in iron deficiency management.
Heme Vs. Non-Heme Iron
Dietary sources of heme iron include red meats, poultry, and seafood, particularly oily fish like sardines and mackerel, and shellfish like oysters and clams. On the other hand, non-heme iron is predominantly found in plant-based foods. Good sources of non-heme iron include legumes, fortified cereals, whole grains, nuts, seeds, and leafy green vegetables.
Iron supplementation encompasses two principal categories: heme iron and non-heme iron.11 Heme iron, derived from hemoglobin and myoglobin found in animal sources, exhibits a superior bioavailability, with absorption percentages ranging from 15-35%.12 This form is less influenced by dietary substances that either enhance or inhibit iron absorption. In contrast, non-heme iron supplements, typically available as ferrous or ferric salts, are widely adopted due to their cost efficiency. These supplements are sourced from plant-based foods, fortified products, and constitute most iron supplements available on the market. Non-heme iron demonstrates variable bioavailability, markedly lower than that of heme iron, with absorption rates documented between 2-20%.11
Absorption enhancers and inhibitors:
The absorption of nonheme iron is modulated by the presence of various dietary components. Compounds such as polyphenols, phytates, calcium, and certain proteins have been identified to impede iron absorption.12 Phytate and polyphenols bind with these components in the gastrointestinal tract, forming complexes that hinder its absorption. Research indicates that calcium influences iron absorption by modulating the activity of iron transporter proteins within enterocytes. Uniquely, calcium has been noted for its ability to reduce the absorption rates of both nonheme and heme iron types. Conversely, ascorbic acid, animal-derived tissues, and specific proteins may enhance the bioavailability of iron. The influence of vitamin C on iron absorption has been demonstrated to be dose-dependent and facilitates increased iron uptake only when both nutrients are ingested together. Meanwhile, the role of dietary fibers in modulating non-heme iron absorption continues to be explored in scientific research.
The most used forms of non-heme iron supplements include:
- Ferrous sulfate: Comprising approximately 20% elemental iron, ferrous sulfate is frequently prescribed for its high bioavailability and affordability. Nonetheless, its utilization can be restricted by gastrointestinal adverse effects.11
- Ferrous gluconate: This compound contains around 12% elemental iron and is generally considered milder on the gastrointestinal system, albeit requiring larger doses to fulfill dietary iron needs.11
- Ferrous Fumarate: With a substantial elemental iron content of about 33%, ferrous fumarate serves as an option for individuals requiring higher iron supplementation.11
- Ferric carboxymaltose: A contemporary choice, ferric carboxymaltose allows for the administration of intravenous iron in high doses with minimal side effects, ideal for severe deficiencies or urgent replenishment needs.9
- Ferrous bisglycinate: This formulation involves the chelation of iron molecules with amino acids, circumventing the challenges of encapsulation instability and the unverified safety associated with nanoparticles, thereby enhancing absorption, and reducing gastrointestinal discomfort. Notably, ferrous bisglycinate exhibits at least a twofold higher bioavailability compared to traditional iron salts. According to a 2023 meta-analysis on 17 randomized controlled trials, ferrous bisglycinate was found to be more efficacious in elevating hemoglobin levels and in mitigating gastrointestinal adverse events among pregnant women in comparison to other iron supplements.10
Consideration for supplement selection:
The selection of an appropriate iron supplement involves several key considerations, which include:
- Gastrointestinal Tolerance: Iron negatively affects the integrity of the intestinal epithelial barrier via oxidative stress and alters the composition and activity of the gut microbiota.6,13 The occurrence of gastrointestinal adverse reactions, such as constipation, nausea, or abdominal discomfort, associated with specific iron supplements, significantly dictates the selection of the supplement form and the dosing schedule.14
- Dietary Habits: For individuals adhering to vegetarian or vegan diets, which exclusively utilize non-heme iron sources, there may be a necessity for supplements of higher bioavailability or the concomitant use of vitamin C to augment iron absorption.15
- Medical Conditions: Certain medical conditions, including inflammatory bowel disease, celiac disease, or history of gastric bypass surgery, can impinge upon the efficiency of iron absorption, necessitating the adoption of bespoke supplementation approaches.16
- Genetic Factors: The presence of genetic disorders such as hemochromatosis, which predispose individuals to iron overload, mandates a customized approach to supplementation, possibly necessitating the reduction or complete avoidance of iron supplementation.17
Ongoing research endeavors are focused on the innovation of new iron supplementation methodologies, such as the engineering of sustained-release formulations and nanoparticles aimed at enhancing bioavailability while minimizing adverse effects. Furthermore, investigations into the influence of the gut microbiome on iron absorption are underway, assessing the potential of prebiotic and probiotic interventions to improve iron status.
Summary
The approach to iron supplementation in women is complex and requires individualization, considering factors such as life stage, dietary patterns, medical history, and tolerance to supplementation. As our comprehension of iron metabolism and its interaction with the gut microbiome deepens, we anticipate the development of increasingly precise and personalized strategies for iron supplementation in women.
In managing iron deficiency anemia, particularly in women, healthcare providers should thoroughly evaluate each patient’s diet, gastrointestinal health, and factors affecting iron absorption before initiating supplementation. Recommendations include advising on combining iron supplements with vitamin C-rich foods to enhance absorption and encouraging the consumption of heme iron from animal proteins, which is more readily absorbed by the body. Additionally, educating patients on timing the intake of iron inhibitors like calcium, and caffeine to minimize their impact. Moreover, evaluating gut health is crucial due to its significant role in iron metabolism. Regular monitoring of iron levels and adjusting dosages or formulations based on patient tolerance and response can optimize treatment. By developing personalized treatment plans that consider factors like pregnancy and specific health conditions, healthcare providers can effectively manage iron deficiency anemia with tailored strategies based on the latest clinical insights.
Citations:
- Zhang AS et al. Iron homeostasis: recently identified proteins provide insight into novel control mechanisms. J Biol Chem. 2009;284(2):711-715.
- Moustarah F et al. Dietary Iron – StatPearls – NCBI Bookshelf (nih.gov). Accessed March 24, 2024.
- Global Nutrition Targets 2025: Policy Brief Series. https://www.who.int/publications-detail-redirect/WHO-NMH-NHD-14.2. Accessed March 24, 2024.
- Daily iron and folic acid supplementation during pregnancy (who.int). Accessed March 24, 2024.
- Jorgensen JM et al. Effects of iron supplementation during lactation on maternal iron status and oxidative stress: a randomized controlled trail. Matern Child Nutr. 2017;13(4):e12394.
- Malesza IJ et al. The dark side of iron: the relationship between iron, inflammation and gut microbiota in selected diseases associated with iron deficiency anemia – a narrative review. 2022;14(17):3478.
- Barton JC et al. Prevalence of iron deficiency in 62,685 women of seven race/ethnicity groups: the HEIRS study. PLoS One. 2020;15(4):e0232125.
- Stoffel NU et al. Oral iron supplementation in iron: how much and how often? Mol Aspects Med. 2020;75:100865.
- Friedrisch JR et al. Intravenous ferric carboxymaltose for the treatment of iron deficiency anemia. Rev Bras Hematol Hemoter. 2015;37(6):400-405.
- Fischer JA et al. The effects of oral ferrous bisglycinate supplementation on hemoglobin and ferritin concentrations in adults and children: a systematic review and meta-analyses of randomized controlled trials. Nutr Rev. 2023;81(8):904-920.
- Iron – Health Professional Fact Sheet (nih.gov). Accessed March 24, 2024.
- Piskin E et al. Iron absorption: factors, limitations, and improvement methods. ACS Omega. 2022;7(24):20441-20456.
- Bloor SR et al. Oral iron supplementation – gastrointestinal side effects and the impact on the gut microbiota. Microbiol Res. 2021;12(2):491-502.
- Tolkien Z et al. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PLos One. 2015;10(2):e0117383.
- Pawlak R et al. Iron status of vegetarian adults: a review of literature. Am J Lifestyle Med. 2018;12(6):486-498.
- Saboor M et al. Disorders associated with malabsorption of iron: a critical review. Pak J Med Scie. 2015;31(6):1549-1553.
- Palmer WC et al. Diagnosis and management of genetic iron overload disorders. J Gen Intern Med. 2018;33(12):2230-2236.
Yekta Dowlati, PhD, serves as the Medical Education Manager at Metagenics. Dr. Dowlati earned her PhD in Medical Sciences from the University of Toronto, along with her MSc in Pharmacology. Her academic credentials also include a BSc in nutrition. She furthered her expertise with a postdoctoral fellowship in Neuropsychopharmacology at the Centre for Addiction and Mental Health in Toronto. Dr. Dowlati’s research portfolio includes multiple clinical trials, and she has contributed to the scientific community through her authorship and co-authorship of articles in prestigious journals, alongside presenting her work at numerous national and international conferences. Before her tenure at Metagenics, she excelled as a senior medical writer and led medical writing teams, demonstrating her passion for learning and education to improve public health. Beyond her professional commitments, Dr. Dowlati cherishes family time, indulging in travel, fitness, and cooking, which speaks to her balanced approach to life.