by Romilly Hodges, MS, CNS
Dynamic Bone
Bone is not a static entity in our body. Our body is continually actively forming bone, as well as breaking it down—a process called bone remodeling. Osteoblast cells are responsible for forming bone matrix, a collagen-proteoglycan mix that can bind mineral salts, mostly as calcium hydroxyapatite. Osteoclast cells are active in resorbing bone matrix by secreting cathepsin K proteases that catabolize bone collagen, and hydrogen ions that lower the surrounding pH and solubilize minerals. A healthy balance between the two opposing activities is what is called ‘homeodynamic’ balance. All the properties of bone are also affected by the integrity of the vasculature and blood flow that delivers important nutrients, removes wastes, and exposes bone cells to any pro-inflammatory and pro-oxidant compounds circulating in the body.
Bone degenerative diseases, such as osteoporosis, reflect disturbances in these mechanisms. For example, the balance between osteoblast and osteoclast activity may shift towards excessive osteoclast (bone resorbing) activity. Poor blood flow to bones creates a localized state of nutrient insufficiency, hypoxia, acidosis, buildup of waste products, and reduced ability to repair bone. Systemic inflammation, derived from non-bone sites such as the gut, can also increase bone loss by reducing osteoblast activity and increasing osteoclast activity.
A Review of Methylation
It is increasingly common to analyze MTHFR and homocysteine status, both potential indicators of methylation capacity in the body, in the context of cardiovascular disease, neurological conditions, infertility and more. Supplemental methylation support is on the radar of many functional and integrative medicine practitioners. Less well-known, however, are the effects of methylation activity on bone health. Yet methylation is also significant for preventing and managing conditions of bone loss.
Methylation is a process that is happening in EVERY cell in the body ALL the time. It is that fundamental and wide-reaching. Methylation is involved in DNA synthesis (cell division and repair), metabolism of hormones, neurotransmitters, histamine and toxins, immune system regulation and cellular energy pathways. Methylation is also the most well-studied form of epigenetic regulation, since methylation ‘marks’ directly on our DNA act to alter the expression of genes. Typically, additional methylation on the promoter region of specific genes serves to silence those genes, or ‘turn them off.’
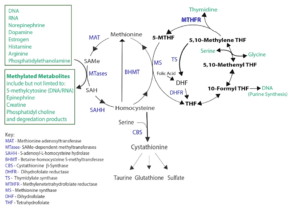
At a biochemical level, methylation involves the addition or formation of methyl groups (CH3) to compounds, altering their structure and function. Methylation reactions almost universally utilize the compound S-adenosylmethionine (SAMe) as the methyl donor. Figure 1 shows how SAMe is formed from methionine and homocysteine, as well as the important role of the MTHFR enzyme.
Figure 1: Formation of SAMe through the methionine and folate cycles
Elevated Homocysteine and Bone Loss
Homocysteine, an amino acid derivative and homologue of the amino acid cysteine, is a functional biomarker indicative of methylation and transulfuration activity. Any deficiency in the activity of the methionine synthase (MS), betaine-homocysteine S-methyltransferase (BHMT) or cystathionine β-synthase (CBS) enzymes can lead to a build-up of homocysteine. These enzymes and their substrates depend on a number of nutrient cofactors, and are also regulated at the epigenetic level by diet and lifestyle. Certain genetic defects in the methylenetetrahydrofolate reductase (MTHFR) enzyme can increase the risk for hyperhomocysteinemia.
Elevated homocysteine has been positively correlated with low bone mineral density (in some but not all studies) and appears to be a risk factor for osteoporotic fracture. Homocysteine also appears to increase osteoclast activity and decrease osteoblast activity, with the potential to negatively alter bone remodeling. In addition, homocysteine has been shown to act via direct mechanisms of bone weakening by inhibit collagen cross-linking enzymes, and indirectly by increasing oxidative stress, which increases bone-matrix degrading matrix metalloproteinases (MMPs). Increased homocysteine also appears to directly decrease bone blood flow.
Supporting methylation pathways can directly reduce homocysteine levels, and may therefore improve markers of bone health.
Methylation tip! Folate and vitamin B12 may be commonly used for methylation support, but there are many other nutrients that can also help such as riboflavin, niacin, vitamin B6, betaine, and zinc. Appropriate, regular exercise plans that incorporate resistance training, but not overtraining, can also reduce homocysteine.
Epigenetic Regulation Requires Adequate Methylation Capacity
Adequate methylation capacity is required to maintain epigenetic methylation status. Global DNA methylation status declines as we age, rendering us more susceptible to diseases of dysregulated epigenetics, including osteoporosis.
Restoring methylation status through dietary intervention has been shown to have a positive (increasing) effect on DNA methylation, demonstrating that epigenetic regulators are indeed modifiable through external factors.
Modulating the SOST (Sclerostin-Producing) Gene
Sclerostin is a signaling protein produced by osteocytes. Through biochemical mechanisms, it inhibits bone formation. It is therefore a target of interest for osteoporosis interventions. In fact, Romosozumab, a drug that is in development for the treatment of osteoporosis, is a sclerostin-inhibiting antibody that is intended to block sclerostin’s normal effects and improve bone formation and density.
Our bodies can also regulate how much Sclerostin is produced through various feedback mechanisms. When bone density falls, as in osteopenia or osteoporosis, the body counteracts that by inhibiting the expression of the SOST gene that produces sclerostin. How does it do this? Through increased methylation on the promoter region of that gene, which acts to turn the gene ‘off.’ Adequate ability to methylate at the DNA level is therefore of utmost importance to preserve the body’s self-balancing mechanisms.
Methylation tip! Dietary and lifestyle methylation support may support SOST inhibition. Physical stimulus such as mechanical loading (think weight-bearing exercise) also inhibits SOST gene expression.
Modulating the RANKL (NF-kB Ligand) Gene
Receptor activator of nuclear factor-kB ligand (RANKL) is a key mediator of osteoclast differentiation, promoting the activation and survival of bone-resorbing cells, including through de-methylation activity. Some osteoporosis treatments, such as Denosumab, are designed to bind and inhibit RANKL. RANKL is opposed by osteoprotegerin (OPG), an active decoy receptor for RANKL which blocks RANKL from stimulating osteoclast formation and activation.
Increased DNA methylation in the RANKL TATA box transcriptional start site is associated with epigenetic silencing of RANKL. Supporting DNA methylation capability may therefore also help to regulate RANKL expression and favorably modulate bone remodeling.
Methylation tip! Curcumin, derived from turmeric spice, has been shown to act epigenetically to increase the ratio of protective osteoprotegerin to RANKL, decreasing osteoclast formation.
Epigenetic Balance Requires Methylation Adaptogens
Supporting adequate methylation capacity is clearly important, but do we need to be cautious about pushing methylation activity too far? The research strongly suggests that we do, especially at the DNA level. Methylation inhibition of gene expression isn’t always favorable and needs to be balanced.
Excessive, dysregulated DNA methylation is associated with diseases including cancer, autoimmunity, atopic disease, Autism and Downs Syndrome. So, at the same time as safely enabling DNA methylation, we should include methylation adaptogens that can appropriately balance DNA methylation activity, even carrying out de-methylation where needed. Again, dietary and lifestyle tools provide the nuanced and sophisticated approach that supports this healthy balance. One example is the plant compound found in cruciferous vegetables: sulforaphane.
Sulforaphane Increases Osteoblast Formation via De-Methylation
Sulforaphane, a compound that occurs naturally in cruciferous vegetables, has been shown to increase the formation of bone-forming cells (osteoblasts) by enhancing de-methylation of DNA in nascent bone cells. This action directs the cell to become a bone-forming, rather than a bone-resorbing cell. Sulforaphane also increases the removal of bone-resorbing cells through regular channels of cellular turnover, a nice one-two punch.
The effects of sulforaphane shift the balance favorably towards increased bone mineralization and density, making supplemental sulforaphane and cruciferous vegetables potentially useful interventions for osteoporosis.
Methylation tip! Sulforaphane-containing cruciferous vegetables include arugula, broccoli, bok choy, Brussels sprouts, cabbage, cauliflower, horseradish, kale, kohlrabi, radish, rutabaga, turnip, wasabi and watercress.
Advanced Methylation Support
Recognition of the potential effects of methylation deficits has led many practitioners, justifiably, to work to supporting methylation status in their patient population. However, scientific understanding of methylation activity in the body only continues to advance, especially at the epigenetic level of DNA methylation.
As practitioners, we need to be concerned with supporting methylation availability and balance, rather than simply pushing methylation reactions forward. Dietary and lifestyle factors provide safe and effective long-term options for methylation support, which can complement or even replace supplement interventions.
Methylation clearly plays an important role in maintaining bone health and there is much we can do to support patients with diseases of bone loss.
Romilly Hodges, MS, CNS, holds a master’s degree in Functional Nutrition from the University of Bridgeport, CT, and is a Certified Nutrition Specialist (CNS). She is passionate about the power of food to nourish and heal the body.
Reposted with permission from Dr. Kara Fitzgerald www.drkarafitzgerald.com