
by Sara Gottfried, MD and Lewis Chang, PhD
Introduction
When I finished my medical training in 1998 at the University of California at San Francisco, I thought I knew everything about vitamin D, particularly vitamin D deficiency and insufficiency. I understood vitamin D was important for the efficient trafficking of calcium in the body, and that helps to keep bones strong, which was important for women’s health over the age of thirty when bone density begins to decline. What I learned in the subsequent twenty years is that vitamin D is both a hormone and a vitamin, and an essential nutrient for more than 400 biochemical jobs in the body. And I learned that what I knew in residency was the tip of the iceberg.
Further, I discovered that I inherited a gene for a faulty vitamin D receptor (VDR), which means my body doesn’t absorb and transport vitamin D well, so I tend to have low levels of vitamin D in my blood. This potentially puts me at greater risk for accelerated bone loss, osteopenia, osteoporosis, multiple sclerosis, and certain malignancies such as colorectal cancer. I’m not that unique in carrying the risky gene, but I’m getting ahead of myself. More later on the gene/environment interactions as they relate to vitamin D status, and whether you should be checking your patients for them. As I continue to identify my own knowledge gaps regarding vitamin D, I have become obsessed with teaching other practitioners and my patients about how persistent vitamin D deficiency and insufficiency continue to be despite the public initiatives to raise awareness. In this series on vitamin D, I want to share with you the importance of vitamin D to your health and that of your patients, starting first in this article with how vitamin D status is determined, how prevalent vitamin D insufficiency and deficiency have been in our population, what factors can affect circulating vitamin D levels, recommendations for your female patients since they are at greater risk of problems, and what proven actions to take to help improve your patients’ vitamin D status.
How is vitamin D deficiency defined?
Serum 25-hydroxyvitamin D [25(OH)D] level is widely used as a biomarker of a person’s vitamin D status, for it is the main circulating form of vitamin D and the best estimator of both endogenous vitamin D synthesis in the skin upon exposure to UV-B light and dietary intake of foods and supplements containing vitamin D.1 Therefore, the diagnosis of vitamin D deficiency is based on the measurement of circulating 25(OH)D levels.
However, there is no consensus among key opinion leaders for what is considered an appropriate cutoff for normal, desirable, and optimal serum 25(OH)D concentrations. As a result, different cut-off points have been used to define hypovitaminosis D (deficiency of vitamin D), ranging from 10 ng/mL (25 nmol/L) to 30 ng/mL (75 nmol/L).2-6
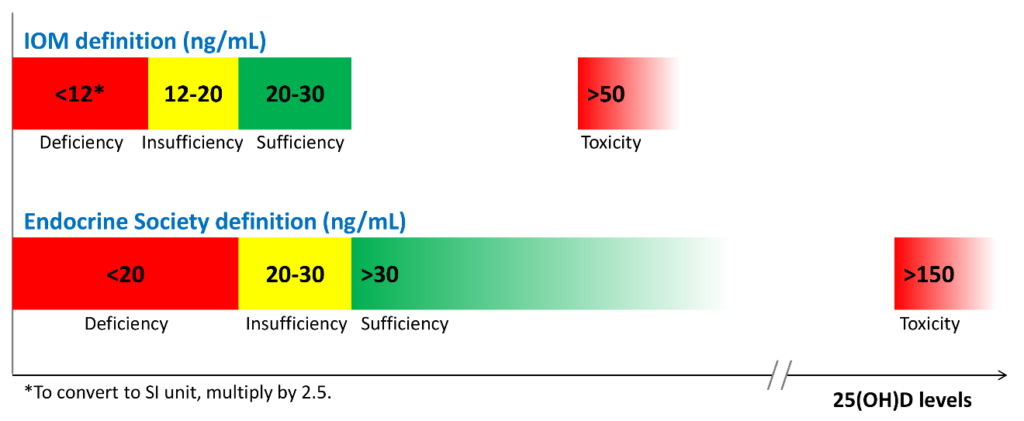
There is no consensus among professional societies, either. In 2010, Institute of Medicine (IOM)—renamed in 2016 as the National Academy of Medicine (NAM)—suggested that circulating 25(OH)D levels need to be at least 20 ng/mL (50 nmol/L) to prevent osteomalacia and maintain bone health in at least 97.5% of the population. Therefore, levels at or higher than 20 ng/mL (>50 nmol/L) are defined as vitamin D sufficient. Levels lower than 20 ng/mL (<50 nmol/L) but above 12 ng/mL (>30 nmol/L) are considered vitamin D insufficient and are inadequate for bone and overall health. Levels below 12 ng/mL (<30 nmol/L) are vitamin D deficient which can cause rickets in infants and children and osteomalacia in adults (Figure 1).7
Conversely, believing the IOM recommendations to be too conservative, the Endocrine Society released its clinical practice guidelines in 2011 in which vitamin D sufficiency, insufficiency and deficiency was defined as circulating 25(OH) levels above 30 ng/mL (>75 nmol/L), between 20-30 ng/mL (50-75 nmol/L), and below 20 ng/mL (<50 nmol/L), respectively (Figure 1).8 These recommended cut-off points are similar to the guidelines released by the Central European Scientific Committee on Vitamin D.9 The Endocrine Society also argued that, since any methodology for 25(OH)D measurement is subject to assay variability, targeting a higher 25(OH)D cut-off value may ensure that an individual meets the requirement for sufficient vitamin D levels with only minimal risk of vitamin D toxicity.8
These conflicting professional recommendations result in constant debate among researchers and confusion among clinicians about where the optimal 25(OH)D levels fall. Nevertheless, it is at least agreed upon that serum vitamin D levels less than 30 ng/mL are definitely insufficient and probably deficient for most people. I advise 50-75 ng/mL.
How prevalent is vitamin D insufficiency and deficiency?
The prevalence varies depending on how vitamin D insufficiency and deficiency is defined. Another important factor that needs to be considered is how 25(OH)D is measured and how differences in the assay methodology (such as assay sensitivity and analytical variability) confound prevalence estimation.
Since 1988, the National Health and Nutrition Examination Surveys (NHANES) have periodically tracked nutrition status of adults and children in the U.S. via examining a nationally representative sample population. These estimates are highly valuable in assessing the trends of vitamin D status over time. However, different assays have been used to determine 25(OH)D levels between 1988 and 2010, from DiaSorin radioimmunoassay to reformulated radioimmunoassay to the more accurate chromatography-based assays. To minimize laboratory method bias and imprecision in these different assays, the Vitamin D Standardization Program—initiated by the US Office of Dietary Supplements in the National Institutes of Health in collaboration with the National Institute of Standards and Technology, Centers for Disease Control and Prevention, and Ghent University—was organized in 2010 to promote standardize 25(OH)D measurement using liquid chromatography-tandem mass spectrometry (LC-MS/MS).11 Also, equations were developed to help standardize data from older radioimmunoassays to LC-MS/MS-equivalent data.
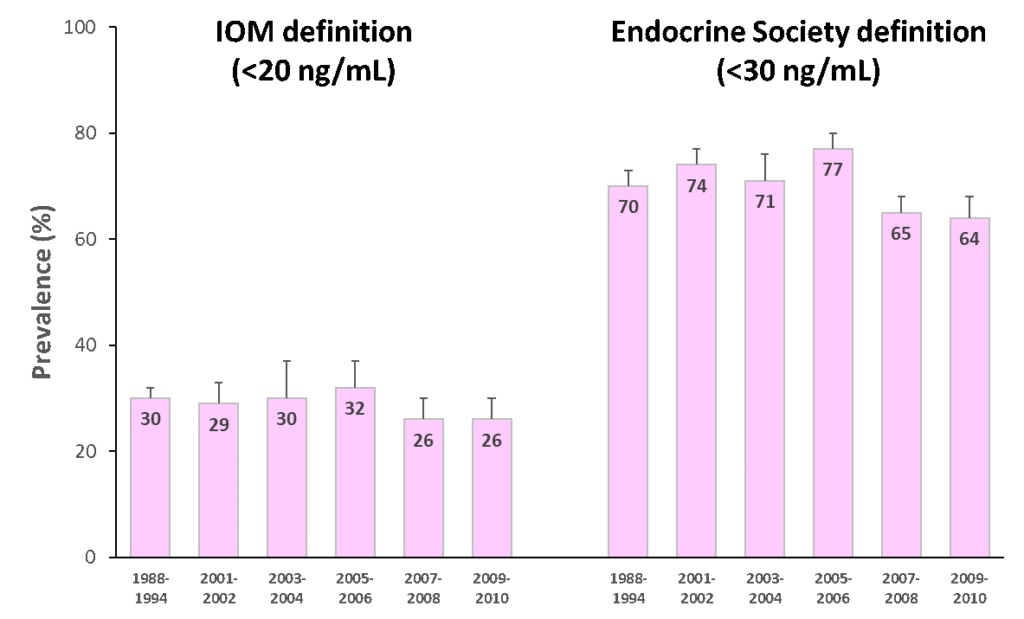
The first paper that reported the temporal trends of vitamin D status in the U.S. using standardized 25(OH)D measurement was published in American Journal of Clinical Nutrition (AJCN) in 2016. They found that the prevalence of IOM-defined vitamin D insufficiency and deficiency (together as one category) was 30-32 percent from 1988 to 2006 and 26 percent from 2007 to 2010 (Figure 2; left). The prevalence was even higher when the Endocrine Society recommended cut-off point was used: 70-77 percent from 1988 to 2006, dropping slightly to 64-65 percent from 2007 to 2010 (Figure 2; right). Also, non-Hispanic blacks and Mexican Americans had much higher prevalence of vitamin D insufficiency and deficiency than non-Hispanic whites.10
Prior to the development of standardizing 25(OH) measurements, an earlier study also had tracked the prevalence trend in the same NHANES cohort and found an increase in vitamin D deficiency over time.1 Now, from the standardized data, we know the prevalence of vitamin D insufficiency and deficiency has been relatively stable. This discrepancy illustrates the importance of method standardization and having data expressed in LC-MS/MS equivalents for the most accurate interpretation of the prevalence trends.
This AJCN report also reported a small increase in vitamin D supplement use (≥ 600 IU/day) among women over time: 2.1% during 1998-1994, increasing to 19% during 2009-2010. Vitamin D supplement users tend to be older (≥40 y/o) and non-Hispanic whites, Still, the majority remained to be non-users; at 70% during 1988-1994 and at 57% during 2009-2010.10
What are the recommended dietary intakes of vitamin D for women?
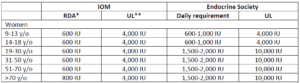
The recommended dietary intakes of vitamin D for women from the IOM and the Endocrine Society are compared in Table 1. IOM recommends 600 IU/day for those who are 9-70 y/o and 800 IU/day for those more than 70 y/o to maintain bone health in at least 97.5% of the population in those age groups. Pregnant and lactating women have the same requirement of 600 IU/day.
The intake levels recommended by the Endocrine Society are higher than IOM’s. For those who are 9-18 y/o, at least 600 IU/day is needed. However, in order to consistently achieve 25(OH)D of 30 ng/mL (75 nmol/L) preferred by the Endocrine Society, 1000 IU/day may be required. For those who are 19 y/o and older, including pregnant and lactating women, at least 1500-2000 IU/day may be required to maintain the blood levels of 25(OH)D to above 30 ng/mL (75 nmol/L).
Table 1. The recommended dietary intakes of vitamin D for women from IOM and the Endocrine Society.
Are dietary intakes of vitamin D sufficient in women in the U.S.?
Overall no. Even among supplement users, the majority of women have insufficient intakes of vitamin D. Read more in this additional article debunking the myths of vitamin D.
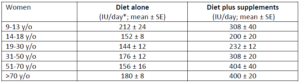
The first study that presented national estimates of vitamin D intakes was conducted by the Office of Dietary Supplements and the National Institutes of Health based on NHANES 2005-2006 data. The average vitamin D intake from diet alone was low in every age group (Table 2); the lowest being those 19-30 y/o (144 IU/day) and the highest 9-13 y/o (212 IU/day). When including vitamin D-containing supplement, vitamin D intakes somewhat increased, particularly among those 51 y/o and older (to approximately 400 IU/day).12 Even so, the intake still fell short of the RDA (600-800 IU/day) and way below the levels recommended by the Endocrine Society (1,500-2,000 IU/day).
Table 2. Mean vitamin D intake from the diet and from diet plus dietary supplements in women based on NHANES 2005-2006 data.12
The low vitamin D intake seen during 2005-2006 was not a single event. A subsequent study that examined data from NHANES 2001-2002, 2003-2004, 2005-2006, and 2007-2008 confirmed the previous pattern: vitamin D intake from diet only remained extremely low across all populations and age groups, falling short of the RDA even when dietary supplement use was included.13 Further, this study identified certain subpopulations that were at greater risks of insufficient vitamin D intakes. When comparing mean intakes by race, African Americans and Mexican Americans had even lower intakes than non-Hispanic whites. When stratifying by annual house income levels, low income was linked to lower vitamin D intakes. Further, those who were affected by obesity had the lowest intake levels compared with those who had normal body weight.13
What about dietary intakes of vitamin D in other countries?
Insufficient intakes of vitamin D is not just a phenomenon in the U.S. In fact, it is a global issue. For example, a study involving populations in representative Western countries (Germany, U.K., and the Netherlands) concluded that vitamin D intakes are below recommendations in a significant part of the population in these countries.14 A more recent overview of vitamin D status globally found that vitamin D deficiency occurred all over the world such as in the Middle East, China, Mongolia, and India.15
How much vitamin D intake is needed to improve vitamin D status in women?
The Women’s Health Initiative Observational Study (WHI-OS), one of the largest cohorts of postmenopausal women in the U.S., has detailed data on vitamin D intake and serum 25(OH)D level, which has allowed the investigators to address this question. Analysis of the data identified a more or less linear relationship between vitamin D intake and serum vitamin D status: every 100 IU increase in total vitamin D intake (diet and supplement combined) was associated with approximately 0.83 ng/mL (2.08 nmol/L) higher serum 25(OH)D levels. Also, total vitamin D intake was the main factor that determined vitamin D status.16
This dose-response observation is consistent with findings from VIDOS (Vitamin D Supplementation in Older Subjects) study, a 1-year randomized trial that investigated vitamin D intakes and serum 25(OH)D levels in healthy postmenopausal women.17 VIDOS study also found that vitamin D intake at 1,600 IU/day could achieve serum 25(OH)D of 30 ng/mL (75 nmol/L) in 97.5% of the participating women. This dosage level is in line with the recommendations from the Endocrine Society.
Is increasing sun exposure alone able to optimize vitamin D status?
Some insights can be gained from a study which investigated serum 25(OH)D levels in a group of healthy men who had just completed a summer season of extended outdoor activity such as landscaping, construction work, farming, or recreation. Compared with the general population, these were people who received some of the highest sun exposure—and with sufficient body surface area exposed and without sunscreen—during the season when UV-B radiation was the strongest. The study found that median serum 25(OH)D concentration in these subjects at late summer reached 48.8 ng/mL (122 nmol/L). However, even with ample summer sun exposure, the median serum 25(OH)D dropped to 29.6 ng/mL (74 nmol/L) by late winter, barely meeting vitamin D sufficiency (30 ng/mL) defined by the Endocrine Society.18
Latitude also determines whether one may obtain sufficient levels of vitamin D via sun exposure. One study compared sun exposure in two different cities, Miami (latitude 26 degrees N) and Boston (latitude 42 degrees N), and found that it’s possible to synthesize vitamin D via skin in all months in Miami but it’s very difficult to do so during winter in Boston.19
Therefore, for a very specific group of people such as landscapers, construction workers and farmers who don’t live in high latitude and don’t use sunscreen, their vitamin D level during summer would be sufficient even without dietary intakes of vitamin D. However, their healthy vitamin D status is not sustainable by end of winter if they don’t have additional sources of vitamin D. For the majority of the population—those who spend most of their daylight hours indoors (e.g., office workers), use sunscreen, have their body surface area covered up (e.g., hat, long-sleeve shirt and pants), or live a sedentary lifestyle, etc.—it is highly unlikely that sun exposure alone can increase serum 25(OH)D to sufficient levels even during summer, let alone the rest of the year.
What are other important factors affecting vitamin D status?
Key non-modifiable factors include age, sex, and race/ethnicity. Although the IOM, the Endocrine Society, and many other professional organizations around the world recommend intake levels based on age and sex, it does not mean that age and sex are the most impactful factors of vitamin D status. Race/ethnicity is another factor that one cannot change, either. However, many factors (besides vitamin D intake) that affect vitamin D status are behavior- and lifestyle-related and therefore modifiable.
A 2018 analysis based on NHANES 2001-2010 data has identified several factors and calculated adjusted prevalence ratios of vitamin D deficiency (<20 ng/mL or 50 nmol/L) and insufficiency (20-30 ng/mL or 50-75 nmol/L) in the general population:20
- Compared with adults with normal body weight, those with obesity had 3.09 times and 1.88 times higher prevalence of vitamin D deficiency and insufficiency, respectively
- Compared with physically active adults, physically inactive adults had 2.00 times and 1.36 times higher prevalence of vitamin D deficiency and insufficiency, respectively
- Compared with during summer, adults in winter had 2.42 times and 1.46 times higher prevalence of vitamin D deficiency and insufficiency, respectively
The link between heavier body weight and poorer vitamin D status is a more recent discovery but from the public health viewpoint its impact can be significant, especially when the obesity epidemic has shown no sign of slowing. Due to higher fat mass, a larger individual may simply need more ingestion or internal synthesis of vitamin D to reach the same concentration as a smaller individual.21 This hypothesis is strengthened by findings from a weight loss intervention trial involving postmenopausal women which demonstrated an increase in serum 25(OH)D levels after weight loss.22
The link between physical activity and vitamin D status may be related to other factors. Decreased physical activity level, especially outdoor types, is associated with reduced sunlight exposure which limits vitamin D synthesis from the skin.8 Lower physical activity may also contribute to weight gain leading to lower serum 25(OH)D levels due to the effect of dilution. Therefore, increasing outdoor physical activity can improve vitamin D status via increased sunlight exposure and improved body weight.
Do genetic factors play an important role in determining vitamin D status?
Several genes encode proteins and enzymes that are involved in vitamin D metabolism. Understandably, genetic variations such as single nucleotide polymorphisms (SNPs) of these genes may influence vitamin D status.
The first genome-wide association study (GWAS) to identify common genetic variants that influence serum 25(OH)D levels was conducted in 2010 by the SUNLIGHT Consortium involving approximately 30,000 Caucasians from Europe, Canada and the U.S.23 This study identified four SNPs that were associated with lower 25(OH)D levels:
- GC; rs2282679. GC gene encodes vitamin D-binding protein.24
- CYP2R1; rs10741657. CYP2R1 gene encodes vitamin D 25-hydroxylase, an enzyme that converts vitamin D into 25(OH)D.25
- DHCR7/NADSYN1; rs12785878. DHCR7 gene encodes 7-dehydrocholesterol reductase, an enzyme that converts 7-dehydrocholesterol (a precursor of vitamin D) to cholesterol.26
- CYP24A1; rs6013897. CYP24A1 gene encodes 24-hydroxylase, an enzyme that is important for the degradation of 25(OH)D and 1,25-dihydroxyvitamin D.27
In 2018, the consortium was expanded to nearly 80,000 individuals (all Caucasians) from 31 epidemiological cohorts.28 The study confirmed the previous four genetic loci and identified two novel loci for serum 25(OH)D levels:
- SEC23A; rs10745742. SEC23A gene encodes a member of SEC23 subfamily which plays a role in promoting endoplasmic reticulum (ER)-Golgi protein trafficking.29
- AMDHD1; rs8018720. AMDHD1 gene encodes an enzyme involved in the catabolic pathway of various amino acids.28
Results from these two studies indicate that, although these common genetic variants are associated with increased risks of vitamin D insufficiency or deficiency, their influence on the variation in serum 25(OH)D levels is relatively small in magnitude when considering all factors. Other studies have drawn similar conclusions.30,31 Vitamin D status is mainly determined by modifiable environmental factors such as dietary intake and sunlight exposure. In terms of determining population-based vitamin D intake recommendations, current evidence does not support the need to take into account genetic information.
However, this is an area of active research, and different genetic variations associated with vitamin D status are being discovered in other studies involving other study populations.32-36 Further, little is yet known on how genetic variants interact with other non-genetic factors. For example, one cohort study involving 1,200 postmenopausal women of European descent found that the association between 25(OH)D levels and certain SNPs in GC and CYP2R1 genes were stronger in summer months but not winter months, or in individuals with dietary intakes of vitamin D above 400 IU/day but not below.37 Also, a randomized controlled trial involving nearly 1,800 adults demonstrated that the increase in 25(OH)D after consumption of 1,000 IU/day vitamin D for 1 year was modified by certain SNPs in CYP2R1, CYP24A1, and VDR gene.38 These gene-environmental interactions and associations, once repeated in larger epidemiological studies and confirmed in large clinical trials, will have important public health implications. It will help determine whether individuals with certain genetic risk factors may indeed require higher amounts of vitamin D to achieve sufficient levels of 25(OH).
Institute of Medicine miscalculation leads to the wrong recommendation of 600 IU/day
As we wrap up, I would like to share with you an interesting article, published in 2014, which was titled “A Statistical Error in the Estimation of the Recommended Dietary Allowance for Vitamin D.” (I have included the hyperlink here in case you are interested in reading the whole open-access article.) In brief, researchers from School of Public Health, University of Alberta (Canada) described how IOM’s RDA for vitamin D was underestimated due to a calculation error. IOM estimated that 600 IU/day of vitamin D would achieve serum 25(OH)D above 20 ng/mL (50 nmol/L) for 97.5% of the population, but the authors re-examined the data and estimated that 600 IU/day of vitamin D would only achieve 10.7 ng/mL (26.8 nmol/L) in 97.5% of the population.39 This means that the dose needed to achieve 20 ng/mL (50 nmol/L) in the majority of the population would have been significantly much higher than 600 IU/day.
Following the publication, another group of researchers verified in another publication that IOM indeed had made a calculation error.40 Miscalculated RDA for vitamin D can have serious public health and clinical implications as the true vitamin D insufficiency and deficiency would have been severely underestimated based on the IOM definition. Many researchers are now calling for the IOM and public health authorities to redefine RDA for vitamin D (closer to what the Endocrine Society has proposed) to allow for appropriate public health and clinical decision-making. In the meantime, I recommend dosing vitamin D to achieve serum sufficiency by starting with the Endocrine Society guidelines, not IOM.
In the next series of articles, I will discuss how vitamin D status is related to women’s health, including current scientific data on the genetic influence of different disease outcomes, as well as common clinical issues we face as clinicians on the front lines.
References
- Ganji V, Zhang X, Tangpricha V. Serum 25-hydroxyvitamin D concentrations and prevalence estimates of hypovitaminosis D in the U.S. population based on assay-adjusted data. J Nutr. 2012;142(3):498-507.
- Ginde AA, Liu MC, Camargo CA, Jr. Demographic differences and trends of vitamin D insufficiency in the US population, 1988-2004. Arch Intern Med. 2009;169(6):626-632.
- Saintonge S, Bang H, Gerber LM. Implications of a new definition of vitamin D deficiency in a multiracial us adolescent population: the National Health and Nutrition Examination Survey III. Pediatrics. 2009;123(3):797-803.
- Mansbach JM, Ginde AA, Camargo CA, Jr. Serum 25-hydroxyvitamin D levels among US children aged 1 to 11 years: do children need more vitamin D? Pediatrics. 2009;124(5):1404-1410.
- Beauchet O, Helard L, Montero-Odasso M, de Decker L, Berrut G, Annweiler C. Hypovitaminosis D in geriatric inpatients: a marker of severity of chronic diseases. Aging Clin Exp Res. 2012;24(2):188-192.
- Rajakumar K, de las Heras J, Chen TC, Lee S, Holick MF, Arslanian SA. Vitamin D status, adiposity, and lipids in black American and Caucasian children. J Clin Endocrinol Metab. 2011;96(5):1560-1567.
- Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53-58.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-1930.
- Pludowski P, Karczmarewicz E, Bayer M, et al. Practical guidelines for the supplementation of vitamin D and the treatment of deficits in Central Europe – recommended vitamin D intakes in the general population and groups at risk of vitamin D deficiency. Endokrynol Pol. 2013;64(4):319-327.
- Schleicher RL, Sternberg MR, Lacher DA, et al. The vitamin D status of the US population from 1988 to 2010 using standardized serum concentrations of 25-hydroxyvitamin D shows recent modest increases. Am J Clin Nutr. 2016;104(2):454-461.
- Sempos CT, Vesper HW, Phinney KW, Thienpont LM, Coates PM, Vitamin DSP. Vitamin D status as an international issue: national surveys and the problem of standardization. Scand J Clin Lab Invest Suppl. 2012;243:32-40.
- Bailey RL, Dodd KW, Goldman JA, et al. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr. 2010;140(4):817-822.
- Wallace TC, Reider C, Fulgoni VL, 3rd. Calcium and vitamin D disparities are related to gender, age, race, household income level, and weight classification but not vegetarian status in the United States: Analysis of the NHANES 2001-2008 data set. J Am Coll Nutr. 2013;32(5):321-330.
- Troesch B, Hoeft B, McBurney M, Eggersdorfer M, Weber P. Dietary surveys indicate vitamin intakes below recommendations are common in representative Western countries. Br J Nutr. 2012;108(4):692-698.
- van Schoor N, Lips P. Global overview of vitamin D status. Endocrinol Metab Clin North Am. 2017;46(4):845-870.
- Cheng TY, Millen AE, Wactawski-Wende J, et al. Vitamin D intake determines vitamin D status of postmenopausal women, particularly those with limited sun exposure. J Nutr. 2014;144(5):681-689.
- Gallagher JC, Sai A, Templin T, 2nd, Smith L. Dose response to vitamin D supplementation in postmenopausal women: a randomized trial. Ann Intern Med. 2012;156(6):425-437.
- Barger-Lux MJ, Heaney RP. Effects of above average summer sun exposure on serum 25-hydroxyvitamin D and calcium absorption. J Clin Endocrinol Metab. 2002;87(11):4952-4956.
- Terushkin V, Bender A, Psaty EL, Engelsen O, Wang SQ, Halpern AC. Estimated equivalency of vitamin D production from natural sun exposure versus oral vitamin D supplementation across seasons at two US latitudes. J Am Acad Dermatol. 2010;62(6):929 e921-929.
- Liu X, Baylin A, Levy PD. Vitamin D deficiency and insufficiency among US adults: prevalence, predictors and clinical implications. Br J Nutr. 2018;119(8):928-936.
- Drincic AT, Armas LA, Van Diest EE, Heaney RP. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity (Silver Spring). 2012;20(7):1444-1448.
- Mason C, Xiao L, Imayama I, et al. Effects of weight loss on serum vitamin D in postmenopausal women. Am J Clin Nutr. 2011;94(1):95-103.
- Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376(9736):180-188.
- Malik S, Fu L, Juras DJ, et al. Common variants of the vitamin D binding protein gene and adverse health outcomes. Crit Rev Clin Lab Sci. 2013;50(1):1-22.
- Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc Natl Acad Sci U S A. 2004;101(20):7711-7715.
- Prabhu AV, Luu W, Li D, Sharpe LJ, Brown AJ. DHCR7: A vital enzyme switch between cholesterol and vitamin D production. Prog Lipid Res. 2016;64:138-151.
- Jones G, Prosser DE, Kaufmann M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): its important role in the degradation of vitamin D. Arch Biochem Biophys. 2012;523(1):9-18.
- Jiang X, O’Reilly PF, Aschard H, et al. Genome-wide association study in 79,366 European-ancestry individuals informs the genetic architecture of 25-hydroxyvitamin D levels. Nat Commun. 2018;9(1):260.
- Amodio G, Venditti R, De Matteis MA, Moltedo O, Pignataro P, Remondelli P. Endoplasmic reticulum stress reduces COPII vesicle formation and modifies Sec23a cycling at ERESs. FEBS Lett. 2013;587(19):3261-3266.
- Berry D, Hypponen E. Determinants of vitamin D status: focus on genetic variations. Curr Opin Nephrol Hypertens. 2011;20(4):331-336.
- Brouwer-Brolsma EM, Vaes AMM, van der Zwaluw NL, et al. Relative importance of summer sun exposure, vitamin D intake, and genes to vitamin D status in Dutch older adults: The B-PROOF study. J Steroid Biochem Mol Biol. 2016;164:168-176.
- Slater NA, Rager ML, Havrda DE, Harralson AF. Genetic variation in CYP2R1 and GC genes associated with vitamin D deficiency status. J Pharm Pract. 2017;30(1):31-36.
- Jolliffe DA, Walton RT, Griffiths CJ, Martineau AR. Single nucleotide polymorphisms in the vitamin D pathway associating with circulating concentrations of vitamin D metabolites and non-skeletal health outcomes: Review of genetic association studies. J Steroid Biochem Mol Biol. 2016;164:18-29.
- Baca KM, Govil M, Zmuda JM, Simhan HN, Marazita ML, Bodnar LM. Vitamin D metabolic loci and vitamin D status in Black and White pregnant women. Eur J Obstet Gynecol Reprod Biol. 2018;220:61-68.
- Touvier M, Deschasaux M, Montourcy M, et al. Determinants of vitamin D status in Caucasian adults: influence of sun exposure, dietary intake, sociodemographic, lifestyle, anthropometric, and genetic factors. J Invest Dermatol. 2015;135(2):378-388.
- Hansen JG, Tang W, Hootman KC, et al. Genetic and environmental factors are associated with serum 25-hydroxyvitamin D concentrations in older African Americans. J Nutr. 2015;145(4):799-805.
- Engelman CD, Meyers KJ, Iyengar SK, et al. Vitamin D intake and season modify the effects of the GC and CYP2R1 genes on 25-hydroxyvitamin D concentrations. J Nutr. 2013;143(1):17-26.
- Barry EL, Rees JR, Peacock JL, et al. Genetic variants in CYP2R1, CYP24A1, and VDR modify the efficacy of vitamin D3 supplementation for increasing serum 25-hydroxyvitamin D levels in a randomized controlled trial. J Clin Endocrinol Metab. 2014;99(10):E2133-2137.
- Veugelers PJ, Ekwaru JP. A statistical error in the estimation of the recommended dietary allowance for vitamin D. Nutrients. 2014;6(10):4472-4475.
- Heaney R, Garland C, Baggerly C, French C, Gorham E. Letter to Veugelers, P.J. and Ekwaru J.P., A statistical error in the estimation of the recommended dietary allowance for vitamin D. Nutrients. 2014;6:4472-4475; Nutrients. 2015;7(3):1688-1690.
Sara Gottfried, MD is a board-certified gynecologist and physician scientist. She graduated from Harvard Medical School and the Massachusetts Institute of Technology and completed residency at the University of California at San Francisco. Over the past two decades, Dr. Gottfried has seen more than 25,000 patients and specializes in identifying the underlying cause of her patients’ conditions to achieve true and lasting health transformations, not just symptom management.
Dr. Gottfried is a global keynote speaker who practices evidence-based integrative, precision, and Functional Medicine. She recently published a new book, Brain Body Diet and has also authored three New York Times bestselling books: The Hormone Cure, The Hormone Reset Diet, and Younger.
Lewis Chang, PhD is Scientific Editorial Manager of R&D at Metagenics. Dr. Chang received his PhD in Nutritional Sciences at University of Washington, along with his MS in Nutrition and Public Health from Teachers College, Columbia University and BS in Pharmacy from National Taiwan University. Prior to joining Metagenics, he conducted dissertation research and completed a research assistantship and postdoctoral fellowship at the Fred Hutchinson Cancer Research Center in Seattle, WA. Dr. Chang has authored or co-authored and managed the publication of over 30 peer-reviewed journal articles and numerous scientific abstracts and posters. He has quite a green thumb, enjoys opera, theater and jazz, and loves cooking, collecting art, and learning to play gypsy jazz guitar.







